Research
The streptomycetes are Gram-positive, soil-dwelling bacteria renowned for their metabolic diversity and their morphological complexity. They produce a vast array of compounds (known collectively as 'secondary metabolites') having profound medical benefits, including anti-cancer agents, immunosuppressants, and more than 80% of commercially important antibiotics. In addition, Streptomyces have a multicellular life-cycle, differentiating into distinct tissue types in response to environmental stimuli, and provide a unique opportunity to explore cell differentiation in a simple, bacterial system.
Intriguingly, differentiation and antibiotic production are intricately connected, and share many regulatory links.
Using genetic, biochemical, cell-biological and post-genomic technologies, the ultimate goals of our research program are:- to understand how cellular differentiation is achieved in a multicellular bacterium
- to understand how newly discovered RNA regulators act to control differentiation, metabolism, and environmental adaptation
- to develop ways of stimulating new antibiotic production, helping to overcome resistance to existing antibiotics and treat infectious disease.
.jpg)
Multicellular Development
The multicellular development of S. coelicolor is a complex process and involves the progression through distinct developmental phases, as shown in the figure above. We are primarily interested in the reproductive phase of growth - a process that culminates in the formation of chains of dormant spores.
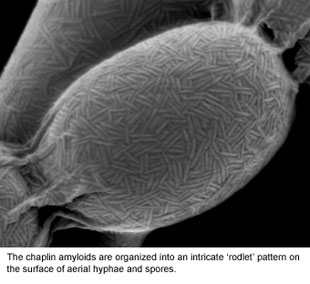
We have identified a family of proteins (the 'chaplins') that coat the outside surface of the cell (see Figure 2) and are absolutely critical for initiating reproductive growth. These 'morphogenetic' proteins interact with each other to form structures known as amyloids. Amyloids are more typically associated with mis-folded proteins in diseases like Alzheimer's and Parkinson's, but for the chaplins, this amyloid structure is key to their functioning. We are working to understand the differences between toxic (disease-causing amyloids), and 'functional amyloids' like the chaplins. To do this, we are probing the underlying genetic and biochemical factors controlling the assembly of these functional amyloids.
We are also interested in understanding cellular dormancy and resuscitation. Many bacterial cells adopt dormant states in as a way of surviving adverse conditions (e.g. starvation or antibiotic treatment). Dormant bacterial populations a therefore major disease reservoirs, with tuberculosis being a prime example - it is estimated that 1/3 of the world's population has a dormant tuberculosis infection! While dormant cells can adopt many forms, they almost all share an altered cell wall structure. We are exploring the importance of cell wall remodelling during both entry into dormancy (sporulation) and return to active growth (resuscitation).
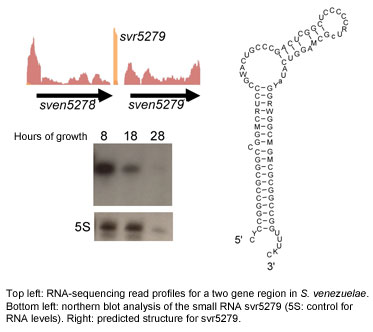
Regulatory RNAs
The last few years have seen non-coding RNAs emerge as important regulators in all organisms. This is an exciting and rapidly evolving field of research, and these newly discovered regulators are predicted to be the "missing link" in many important cellular pathways. Because this is such a new field, there is much that is still unknown about their prevalence, their action and their roles in the cell.
We are currently mining Streptomyces genomes for non-coding RNAs, using bioinformatic strategies, alongside genetic, genomic and biochemical tools. Our long-term aim is to define novel RNA regulatory networks, and integrate RNA regulation into known regulatory cascades controlling development and antibiotic production. This work will provide key insights into the biology of these medically and developmentally important bacteria.
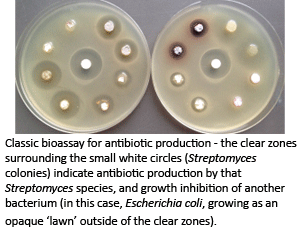
Stimulating antibiotic production
Streptomyces have long been known to be major producers of antibiotics; however, the advent of genome sequencing has revealed that they have the genetic capacity to produce far more (5-10x more!) antibiotic-like molecules than have ever been detected. We have developed powerful genetic approaches that effectively stimulate the production of these 'cryptic' (never-before-been-seen) antibiotics, and are working to mine this completely untapped metabolic reservoir to identify new antibiotics that can be used in the fight against infectious disease.